2021年5月10日,安进宣布向FDA递交TSLP抗体Tezepelumab的上市申请,用于治疗重度哮*[1]。早在2020年11月10日,安进/阿斯利康联合宣布其TSLP抗体Tezepelumab治疗重度哮*的三期临床NAVIGATOR获得成功;尽管后续SOURCE试验结果显示:与安慰剂相比,Tezepelumab在未能达到每日口服糖皮质激素(OCS)剂量在统计上显著减少的主要终点,但Tezepelumab的其他疗效指标与的前期研究结果一致,包括注册III期NAVIGATOR研究。此外,Tezepelumab的安全性结果也与之前的研究一致[2]。
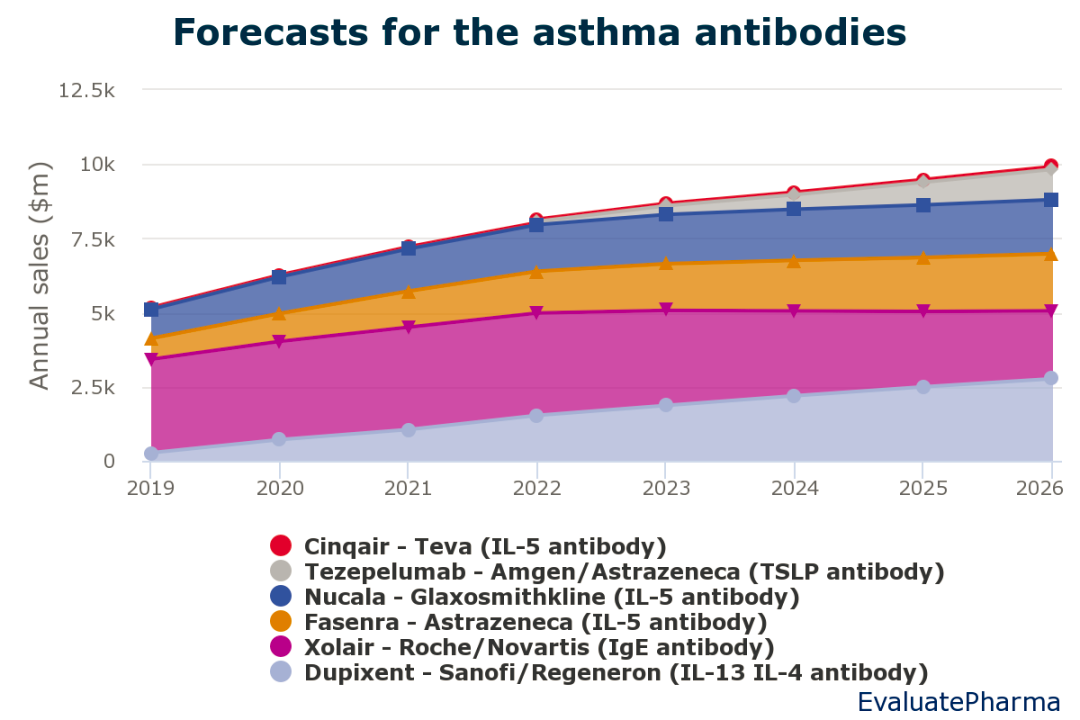
根据EvaluatePharma的数据显示,预计Tezepelumab市场上市后,将会在2026年突破十亿美元年销售额大关,达到10.21亿美元(Fig.1)[3]。
TSLP R:TSLP:IL-7Rα三元复合物
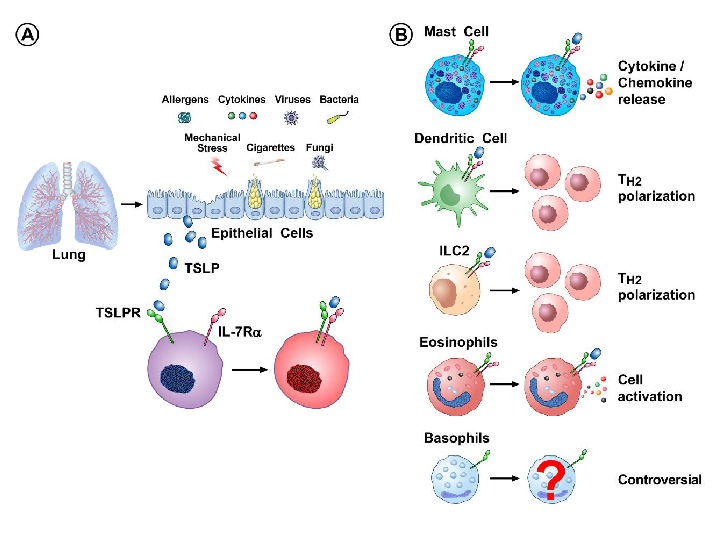
Fig. 2 A. TSLP is expressed predominantly by lung epithelial cells. B. Human mast cells express both TSLPR and IL-7Rα and TSLP induces the release of cytokines/chemokines.
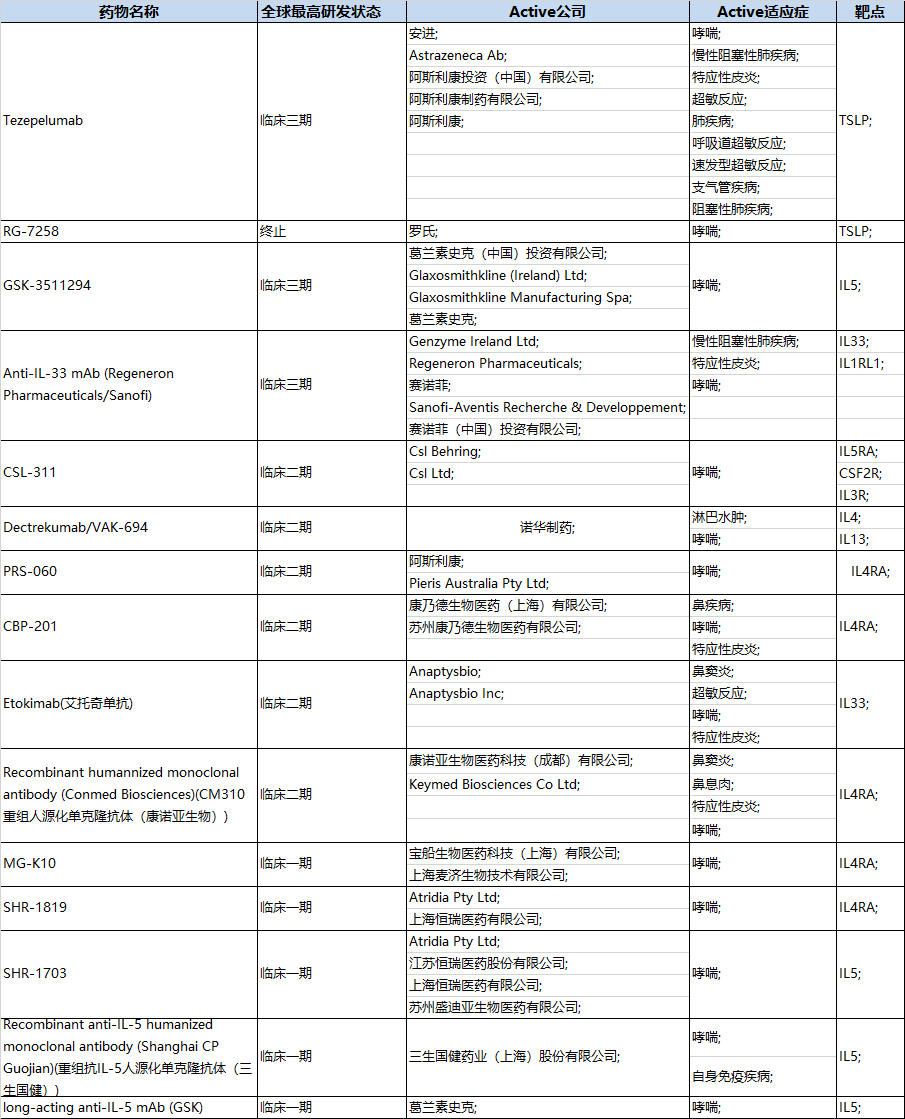
同在Th2通路中的哮*靶点IL-4、IL-5、IL-13、IL-33作用也已经被验证具有临床治疗价值。以赛诺菲/再生元的IL-4R抗体Dupixent为例,该抗体适应症为湿疹与哮*,上市以来销售额一路攀升,2020年销售额40亿美元。目前哮*相关药物临床实验主要包括IL-4、IL-5、IL-13、IL-33与TSLP靶点。(见下表)
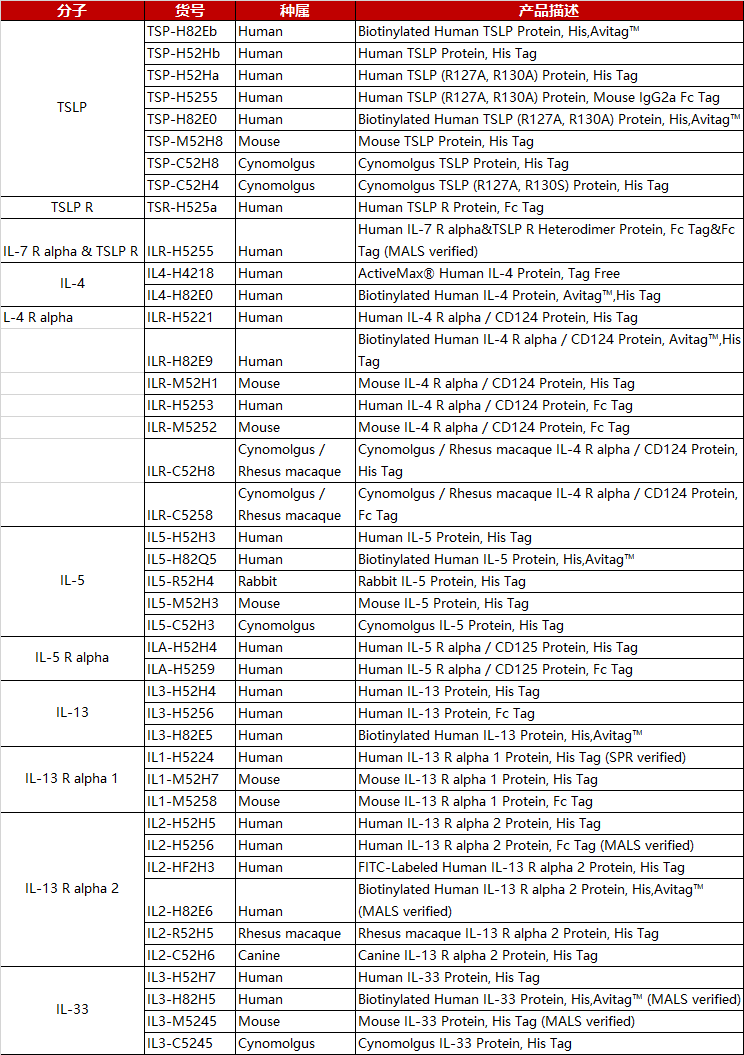
验证数据
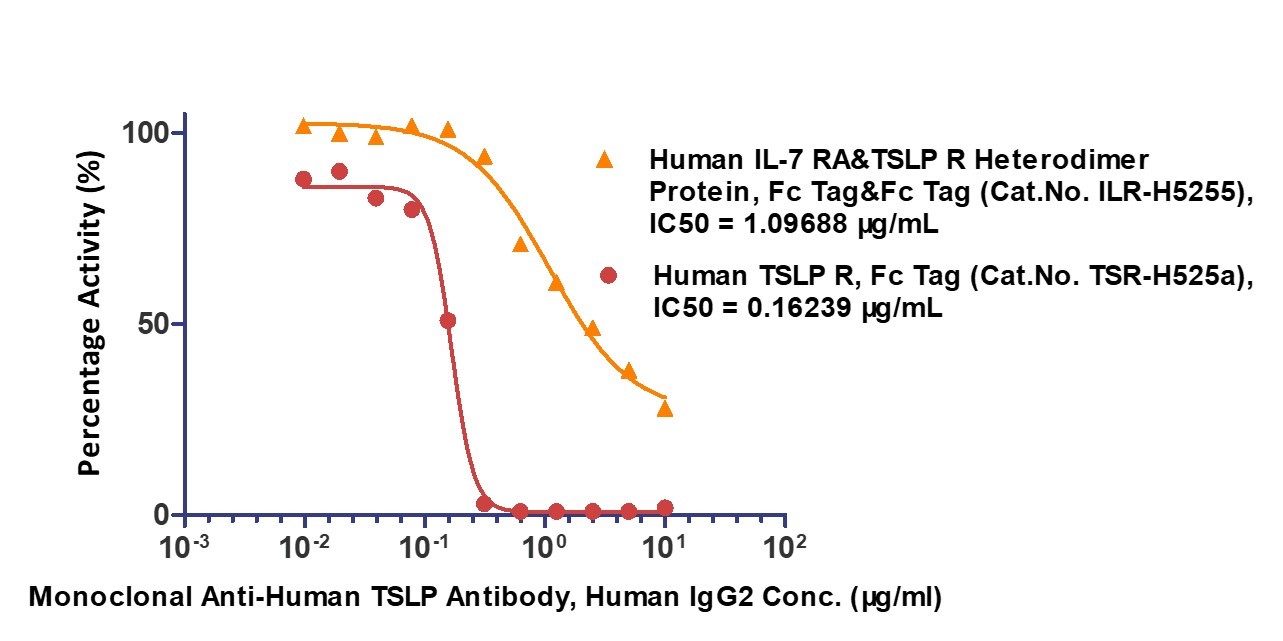
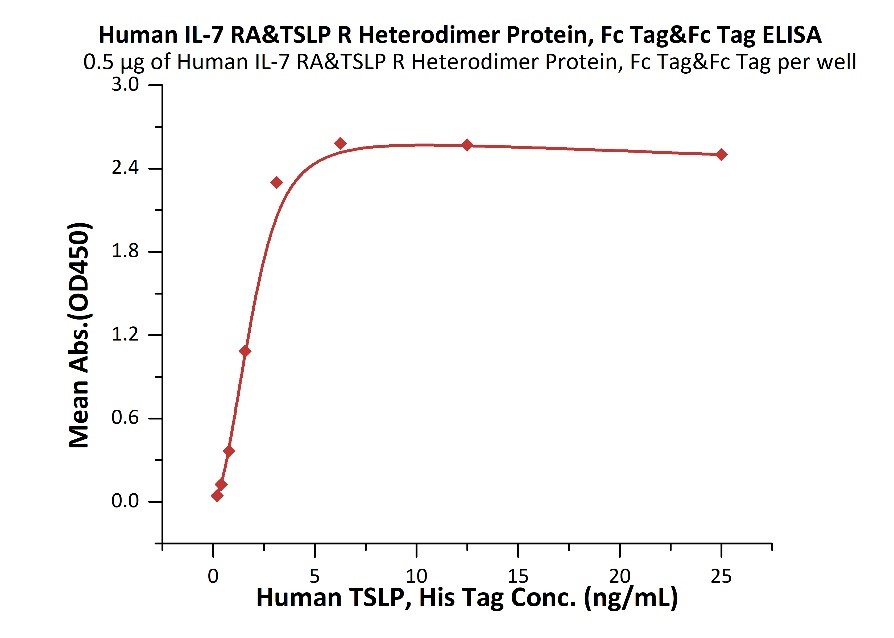
Serial dilutions of Anti-Human TSLP Antibody, Human IgG2 were added into Human IL-7 R alpha & TSLP R Heterodimer Protein, Fc Tag & Fc Tag (MALS verified) (Cat. No. ILR-H5255) and Human TSLP R, Fc Tag (Cat. No. TSR-H525a): Biotinylated Human TSLP Protein, His,Avitag™ (Cat. No. TSP-H82Eb) binding reactions. The half maximal inhibitory concentrations (IC50) of Human IL-7 R alpha & TSLP R Heterodimer Protein, Fc Tag & Fc Tag (MALS verified) and Human TSLP R, Fc Tag are 1.09688 μg/mL and 0.16239 μg/mL respectively.
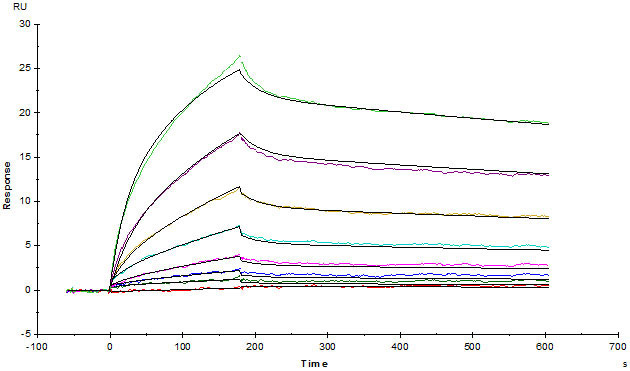
Biotinylated Human SPR
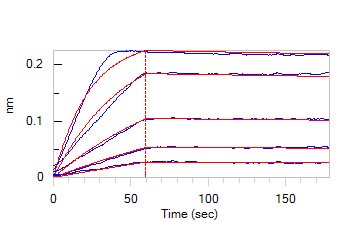
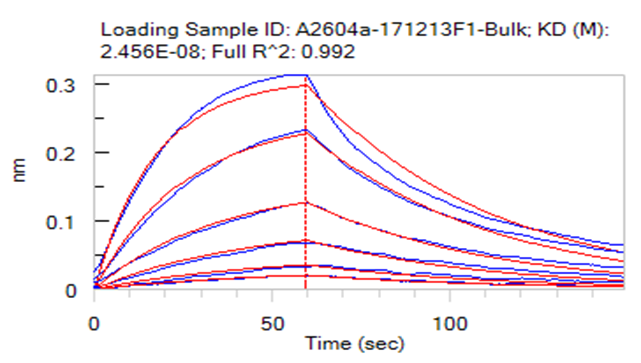
Loaded Human IL-7 RA&TSLP R Heterodimer Protein, Fc Tag&Fc Tag (Cat. No. ILR-H5255) on Protein A Biosensor, can bind Human TSLP, His Tag (Cat. No. TSP-H52Hb) with an affinity constant of 134 pM as determined in BLI assay (ForteBio Octet Red96e).
您可通过以下方式联系到ACROBiosystems:
邮件:inquiry@acrobiosystems.com
电话:15117918562

(备注:姓名+公司)
参考资料
1.https://www.amgen.com/newsroom/press-releases/2021/05/amgen-announces-tezepelumab-biologics-license-application-submitted-to-u-s--fda
2.https://www.astrazeneca.com/media-centre/press-releases/2020/update-on-source-phase-iii-trial-for-tezepelumab-in-patients-with-severe-oral-corticosteroid-dependent-asthma.html
3.https://www.evaluate.com/vantage/articles/news/trial-results/source-flop-raises-tezepelumab-questions
4.Marone G, et al. Expert Opin Investig Drugs. 2019. PMID: 31549891 Review.
5.Varricchi G, Pecoraro, A, Marone, G, et al. Thymic Stromal Lymphopoietin Isoforms, Inflammatory Disorders, and Cancer. Front Immunol. 2018; 9: 1595.