嵌合抗原受体T(CAR-T)细胞疗法是一种基于体外改造T细胞,使其表面表达特异性识别肿瘤表面抗原的受体片段的癌症免疫疗法,改造后的T细胞输入患者体内,不需要抗原递呈细胞(APC)的帮助,直接靶向体内癌细胞并发挥免疫杀伤作用。
对于CAR-T细胞来说,发挥肿瘤杀伤作用的有效成分是CAR阳性的T细胞,CAR-T细胞产品的包装规格及临床使用剂量是以CAR-T阳性细胞数表示的,因此,CAR转染阳性率是CAR-T质量控制的必检项,监管部门建议应采用流式细胞法检测CAR转染阳性率。目前有针对CAR不同结构区域的检测方法,包括针对CAR抗原结合位点的,比如CD19抗原,或针对轻链或铰链区的抗Fab抗体或Protein L蛋白,其中,针对抗原结合部位的CAR阳性率检测方法因其具有更好专属性而被广泛使用。
ACROBiosystems作为专注于医药研发领域的蛋白供应商,利用专业的蛋白研发平台、蛋白标记平台、稳定株开发平台和流式细胞分析平台,开发了一系列包括非标记、生物素标记、荧光标记等多种形式的CAR-T靶点蛋白以及配套的流式细胞法检测CAR阳性率的protocol,以助力CAR-T的研发,加速CAR-T研发的进程。目前产品已覆盖BCMA,CD19,ROR1,EGFRVIII等20余个CAR-T热门靶点.
CAR-T阳性率检测策略及蛋白产品设计
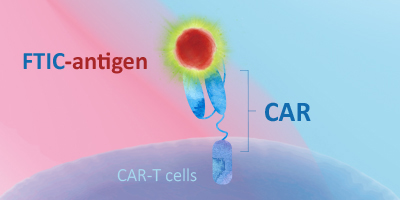
直接检测法 —— 荧光标记蛋白
产品特点
- 靶抗原预先标记了荧光素基团
- 可简化检测流程,节省检测时间
- 可避免因二抗交叉反应而引起的非特异性背景
> FITC-labeled (点击分子即可查看产品详情)-> 独有产品
| BCMA | CD19 | CD22 | GPC3 | MSLN | Protein L |
> PE-labeled (点击分子即可查看产品详情)-> 独有产品
| MSLN |
> 应用案例
使用FITC标记CD19蛋白检测Anti-CD19-CAR表达阳性率

293 cells were transfected with anti-CD19-scFv and RFP tag. 2e5 of the cells were stained with B. FITC-labeled Human CD19 (20-291) (Cat. No. CD9-HF2H2, 10 µg/ml) and C. FITC-labeled protein control. A. Non-transfected 293 cells and C. FITC-labeled protein control were used as negative control. RFP was used to evaluate CAR (anti-CD19-scFv) expression and FITC was used to evaluate the binding activity of FITC-labeled Human CD19 (20-291) (Cat. No. CD9-HF2H2).
> 如需了解更多此产品信息,可点击:ACROBiosystems官网,输入货号,即可查看产品详情,也可直接拨打:400-682-2521进行咨询
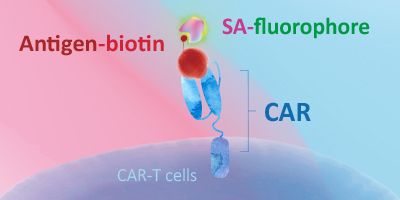
基于生物素-亲和素的检测法 —— 生物素标记蛋白
产品特点
- 靶抗原预先标记了生物素,随后可使用荧光标记的链霉亲和素检测
- 链霉亲和素对生物素具有极高的亲和力,两者的结合具有高度特异性
- 可高灵敏度和高特异性地检测CAR阳性表达率
> AvitagTM biotinylated proteins -> Specially designed
| BCMA | CD22 | CD30 | CD33 | CD38 | CD70 |
| EGF R | EGFRVIII | EpCAM | FOLR1 | GPC3 | HER2 |
| HGFR | MSLN | ROR1 |
> Chemically biotinylated proteins -> Specially designed
| CD19 | EpCAM | HER2 | MSLN | Protein L |
> 应用案例
使用生物素标记BCMA蛋白检测Anti-BCMA-CAR表达阳性率
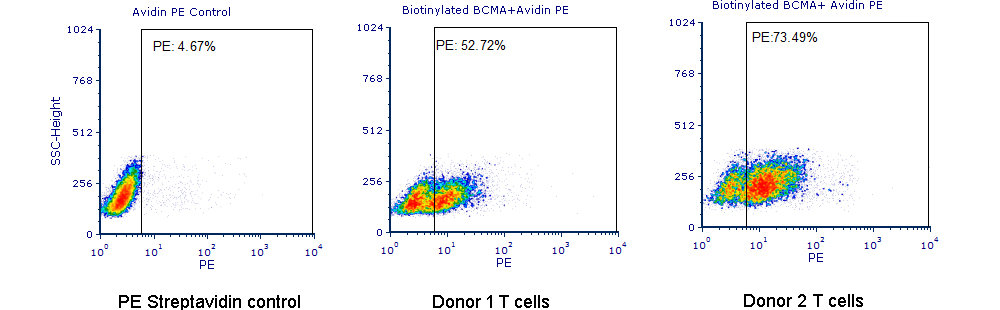
Human T cells were transfected with anti-BCMA CAR and cultured for 3 days. Three days post-transfection, 1e6 cells were first incubated with 50 µl biotinylated human BCMA protein (Cat. No. BC7-H82F0, 8 µg/ml), washed and then stained with PE Streptavidin and analyzed by flow cytometry. (Data are kindly provided by PREGENE Biopharma)
> 如需了解更多此产品信息,可点击:ACROBiosystems官网,输入货号,即可查看产品详情,也可直接拨打:400-682-2521进行咨询
间接检测法 —— 非标记蛋白
产品特点
- 靶抗原带有融合表位标签,随后可使用荧光标记的表位标签抗体作为二抗进行检测
- 可高灵敏度地检测CAR阳性表达率
- 有可能产生因二抗交叉反应引起的非特异性背景
> Fc-tag fusion proteins -> Specially designed
| BCMA | CD19 | CD30 | CD33 |
| CD38 | CD70 | EGFR | FOLR1 |
| GPC3 | HER2 | HGFR | IL13R |
| MSLN | MUC1 | ROR1 | EpCAM |
> His-tag fusion proteins -> Specially designed
| BCMA | CD19 | CD123 | CD138 | CD22 | CD30 |
| CD33 | CD38 | CD70 | CAIX | EGFR | EGFRVIII |
| EpCAM | FOLR1 | GPC3 | HER2 | HGFR | MSLN |
| ROR1 |
> All fusion proteins -> Specially designed
| BCMA | CD19 | CD123 | CD138 | CD22 | CD30 |
| CD33 | CD38 | CD70 | CAIX | EGFR | EGFRVIII |
| EpCAM | FOLR1 | GPC3 | HER2 | HGFR | IL13RA2 |
| MSLN | MUCI | ROR1 |
> 应用案例
使用Human CD19, Fc Tag蛋白检测Anti-CD19-CAR表达阳性率
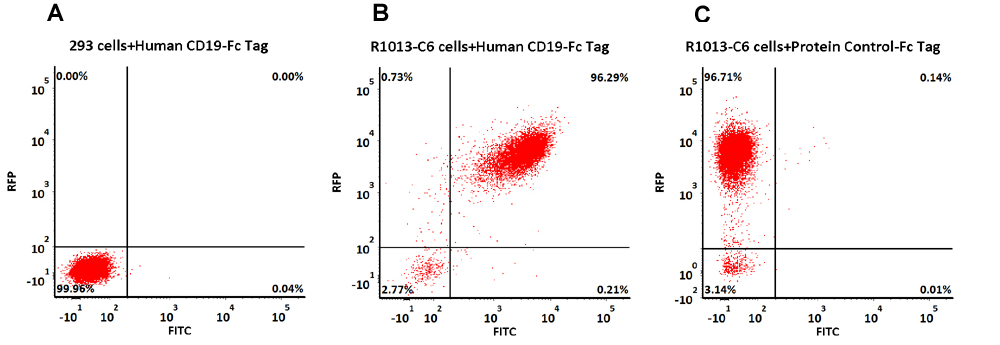
293 cells were transfected with FMC63-scFv and RFP tag. 2e5 of the cells were first stained with B. Human CD19 (20-291) Protein, Fc Tag, low endotoxin (Super affinity) (Cat. No. CD9-H5251, 3 µg/ml) and C. Human Fc Tag Protein Control, followed by FITC-conjugated Anti-human IgG Fc Antibody. A. Non-transfected 293 cells and C. Human Fc Tag Protein Control were used as negative control. RFP was used to evaluate CAR (anti-CD19-scFv) expression and FITC was used to evaluate the binding activity of Human CD19 (20-291) Protein, Fc Tag, low endotoxin (Super affinity) (Cat. No. CD9-H5251).
> 如需了解更多此产品信息,可点击:ACROBiosystems官网,输入货号,即可查看产品详情,也可直接拨打:400-682-2521进行咨询
更多CAR-T相关内容
> 点击查看更多CD19详细信息——高亲和力CD19蛋白,专为检测CD19-CAR-T而设计
