CD19是当前细胞免疫疗法中最具研究热度的靶点之一,已有多款CD19CAR-T细胞疗法获批上市。目前,关于靶向CD19的细胞疗法除了自体CAR-T疗法研究以外,还包括通用型CAR-T、多靶点CAR-T、CAR-NK疗法以及免疫检查点联合疗法等多种新型疗法模式,不断给肿瘤患者带来新希望。为助力CD19新型细胞疗法的开发,ACROBiosystems专门提供包括CD19蛋白、核酸酶、GMP细胞因子、类CD19CAR细胞株、抗FMC63抗体等在内的七大系列核心研发工具,可应用于早期研发、质量控制、工艺生产以及临床等研究阶段,全面支持CD19细胞疗法开发,加速药物研发进程。
★ 真核表达系统,保持蛋白天然构象
★Alexa Fluor 647\555\488、APC、PE、FITC等多种标记类型
★ 多种属可供选择(人/鼠/猴/犬等)
★ 可用动物免疫、抗体筛选、CD19 CAR表达检测
★ 完成FDA DMF备案,支持IND、BLA申报
>>> 纯度大于90%
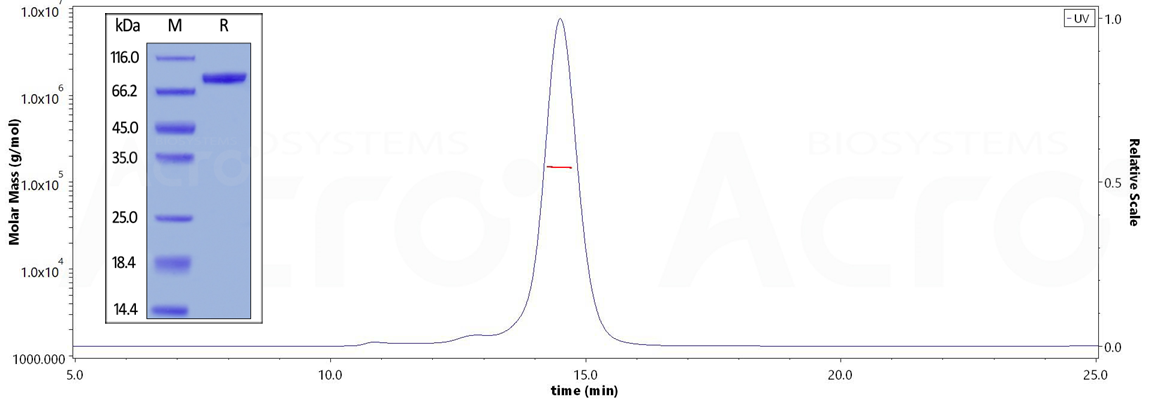
The purity of Human CD19 (20-291), Fc Tag (Cat. No. CD9-H5251)is more than 90% and the molecular weight of this protein is around140-160 kDa verified by SEC-MALS.
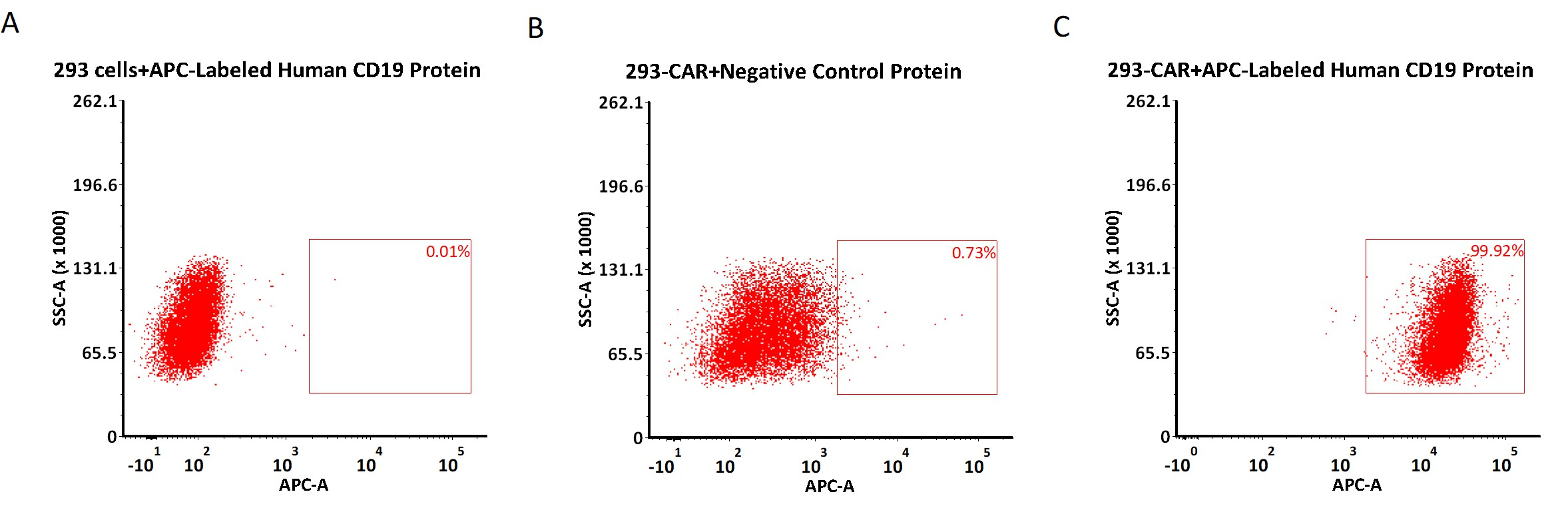
>>> APC标记CD19蛋白可高特异检测CD19 CAR表达
全能核酸酶
GENIUS™Nuclease全能核酸酶,具有纯度高、酶活高、特异性强、反应条件更广泛等产品特性,可高效去除慢病毒纯化过程中的残留宿主核酸;经过无菌、支原体、内毒素、外源病毒等检测控制,可确保产品的安全性;已完成FDA DMF备案,支持临床/上市申报。
更有高质量的全能核酸酶残留检测试剂盒GENIUS™Nuclease ELISA Kit,点击了解>>
Cas酶
高酶活经过体内/体外实验验证:体外切割效率>90%
SDS-PAGE& MALS检测纯度>90%;无RNA酶残留
可用于CD19通用型CAR-T疗法中基因的敲除或敲入
>>> GENIUS™ Nuclease, premium grade比竞品具有更高的活性(>1.2 x 10e6 units/mg)
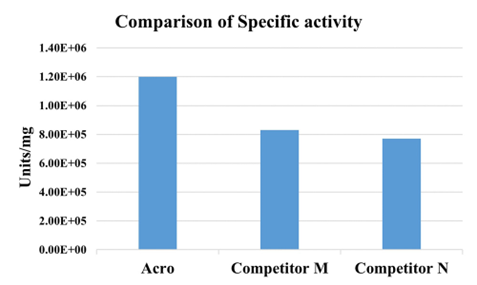
Specific activity for GENIUS™ Nuclease, premium grade is measured understandard assay conditions. The specific activity of GENIUS™Nuclease, premium grade is >1.2 x 10e6 units/mg protein. One unitwill digest sonicated salmon sperm DNA to acid-solubleoligonucleotides equivalent to a ΔA260 of 1.0 in 30 min at pH 8.0 at37 ℃, which corresponds approximately to complete digestion of 37μg DNA. Note that 1 KU=1000 units.
>>> Cas12a的体外DNA底物切割效率>90%,与竞品相当
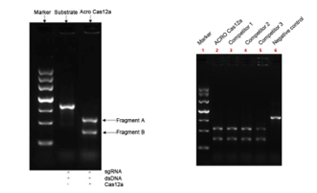
Measured by its ability to cleave a targeted DNA substrate. Cas12a achieves>90% substrate cleavage, comparable to competing products
高质量的CD3/CD28抗体偶联磁珠产品,5.5μm大小,可更好的模拟APC,超低内毒素(<2EU/mg),对T细胞无伤害,可放心用于T细胞的激活扩增。
>>> ACRO Anti-CD3/CD28 Antibody-coupled Magnetic Beads (Cat. No. MBS-C001) 与竞品分别激活T细胞,激活能力水平基本一致
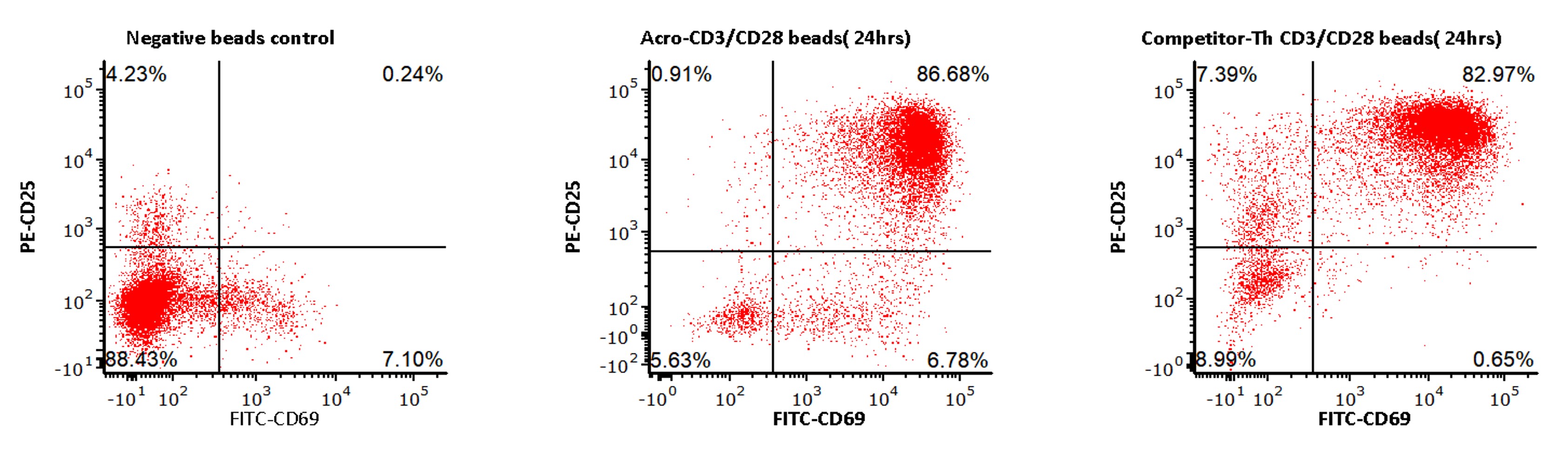
The purified human T cells were activated using ACRO Anti-CD3/CD28Antibody-coupled Magnetic Beads (Cat. No. MBS-C001)and competitor’s beads respectively at a ratio of 1:1beads-to-cells for 24 hours with RPMI1640 supplemented with 10% ofFBS. Cells were fluorescently stained using PE labeled anti-humanCD25 antibody and labeled FITC anti-human CD69 antibody and analyzedby flow cytometry.
在严格GMP 管理体系下开发的GMP级别IL-7,IL-15,IL-21等细胞因子,活性经WHO标品标定,稳定性好,批间一致性高,可应用于工艺生产、临床研究阶段的细胞扩增培养。
>>> 高生物活性
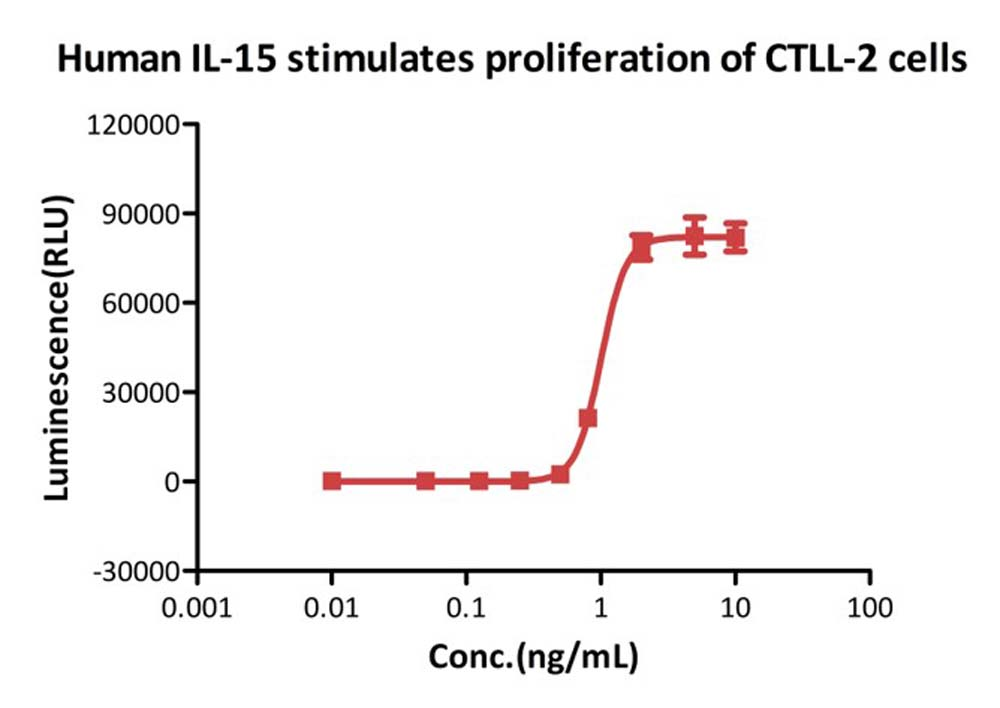
刺激CTLL-2 细胞增殖实验结果显示,GMP Human IL-15 (GMP-L15H13)的细胞活性高于0.8 ⅹ10^7 IU/mg(经WHO Human IL-15标品校准,NIBSC 代码:95/554)
>>> 高批间一致性
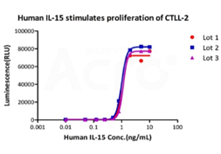
GMP Human IL-15 (GMP-L15H13)不同批次的细胞活性保持一致,具有高批间一致性。
Anti-CD19(FMC63) CAR细胞株,scFv为FMC63来源,细胞活率大于90%,无菌、无真菌、无支原体,多次传代后可保持性能稳定,适用于CAR检测时的CAR阳性对照细胞。
>>> Anti-CD19(FMC63) CAR 细胞株可特异性结合CD19蛋白
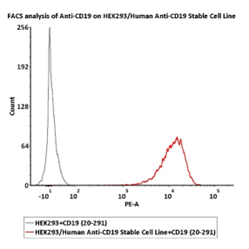
FACS assay shows that PE-Labeled Human CD19 (20-291), His Tag can bind toHEK293/Human Anti-CD19 Stable Cell Line. HEK293/Human Anti-CD19Stable Cell Line was red line, Negative control HEK293 cells was greyline (QC tested).
★ 可特异性结合Anti-CD19(FMC63) CAR的抗原识别表位,具有中和活性;
★ APC\PE\FITC等多种荧光标记类型;
★ 经CD19 类CAR细胞流式批检、PBMC批检放行;
★ 解决CD19 CAR-T临床样本组分复杂、CAR-T细胞含量较低、FACS非特异背景强等问题;
★ 专为FMC63 scFv来源的CD19 CAR细胞PK/ADA Assay而设计
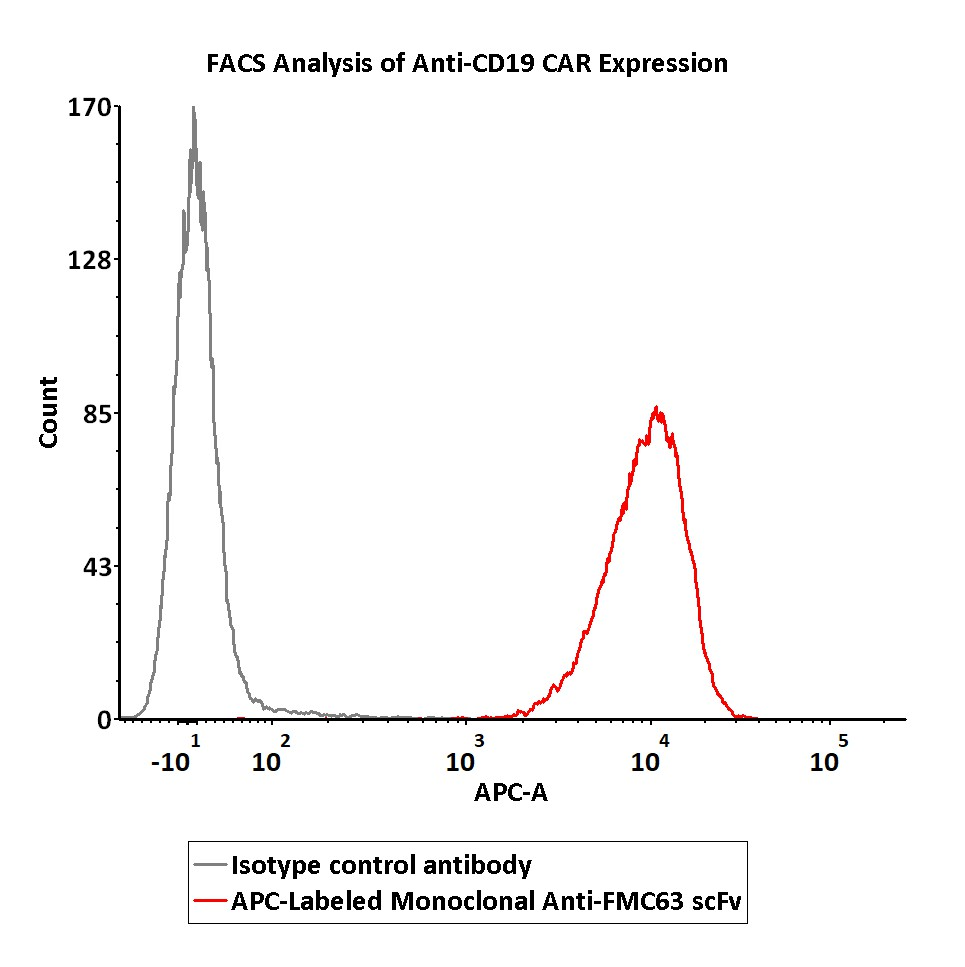
5e5 of anti-CD19 CAR-293 cells were stained with 100 μL of 1:50 dilution(2 μL stock solution in 100 μL FACS buffer) of APC-LabeledMonoclonal Anti-FMC63 scFv Antibody, Mouse IgG1 (Y45) (Cat. No.FM3-AY54P1)and isotype control antibody respectively. APC signal was used toevaluate the binding activity (QC tested).
>>> Screening assay-桥接式ELISA
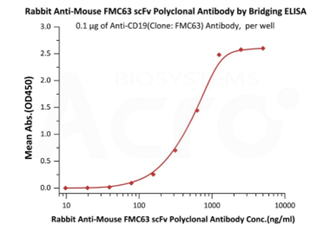
兔源多克隆Anti-MouseFMC63 scFv Antibody (Cat. No. FM3-S93)可分别与anti-CD19antibody (Clone: FMC63)、Biotinylatedanti-CD19 antibody (Clone: FMC63)结合,适用于桥接式ELISA方法开发。
>>> Neutralizing antibody detection-竞争配体结合试验
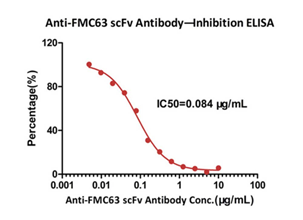
具有中和活性的单克隆Anti-FMC63scFv Antibody, Mouse IgG1 (Y45) (Carrier-free) (Cat. No.FM3-Y45A1)可特异性抑制FMC63scFv与CD19的结合,可用于检测临床样本中具有中和活性抗药性抗体含量。
ACROBiosystems提供一系列细胞因子定量检测试剂盒,具有高灵敏、高特异、高批间一致等特性,可用于靶向CD19细胞药物开发过程中的细胞因子残留检测、细胞功能评价、CRS风险评估等研究。
>>> Human Interferon-γ(IFN-γ) ELISA Kit可高灵敏检测人干扰素-γ的含量
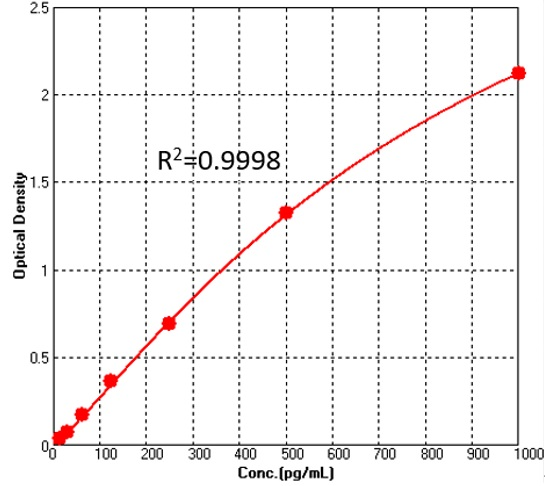
通过3次独立实验,采用20个零标准品浓度OD的平均值加上两倍SD,计算最低可检测浓度,测得选用Cat.No. CRS-A001检测人干扰素-γ的最低可检测浓度为3.072pg/mL。
ACROBiosystems百普赛斯致力与您在细胞和基因治疗的发展浪潮中并肩前行,我们提供包括CAR靶点蛋白、TCR靶点蛋白、StarStaining新一代荧光标记蛋白、GMP级别细胞因子、CD3/CD28抗体偶联磁珠、Cas酶、全能核酸酶、细胞因子检测试剂盒等在内的一系列支持细胞和基因治疗药物开发的相关产品,整套解决方案陪伴您从药物早期发现到临床研究的每一个阶段。

ACROBiosystems
inquiry@acrobiosystems.com
15117918562
(备注:姓名+公司)