乙酰胆碱测试盒/乙酰胆碱测试盒
产品名称: 乙酰胆碱测试盒/乙酰胆碱测试盒
英文名称: EnzyChrom™ Acetylcholine Assay Kit
产品编号: EACL-048, EACL-100
产品价格: 0
产品产地: 美国
品牌商标: BioAssay Systems(美国博世)
更新时间: null
使用范围: null
- 联系人 :
- 地址 : 成都市新都区泰兴普河工业园105号
- 邮编 :
- 所在区域 : 四川
- 电话 : 点击查看
- 传真 : 点击查看
- 邮箱 : support@basbioinc.com
|
Description acetylcholine is a neurotransmitter produced in acetylcholinergic neurons. It plays important roles in skeletal muscle movement, regulation of smooth and cardiac muscles, as well as in learning, memory and mood. BioAssay Systems method provides a simple, direct and high-throughput assay for measuring acetylcholine in biological samples. In this assay, acetylcholine is hydrolyzed by acetylcholinesterase to choline which is oxidized by choline oxidase to betaine and H2O2. The resulting H2O2 reacts with a specific dye to form a pink colored product. The color intensity at 570nm or fluorescence intensity (530/585 nm) is directly proportional to the acetylcholine concentration in the sample.
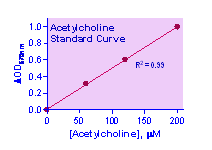
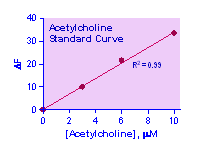
Key features Use 20 mL samples. Linear detection range: colorimetric assay 10 to 200 mM, fluorimetric assay 0.4 to 10 mM acetylcholine.
Applications Assays: acetylcholine in biological samples such as serum, plasma, urine, saliva, milk, tissue, and cell culture. Drug Discovery/Pharmacology: effects of drugs on acetylcholine metabolism.
Kit Contents Assay Buffer: 10 mL ACHE Enzyme: 120 mL Enzyme Mix: 120 mL Dye Reagent: 120 mL Standard: 400 mL 2 mM acetylcholine
Storage conditions. The kit is shipped on ice. Store all components at -20°C. Shelf life of three months after receipt.
Precautions: reagents are for research use only. Normal precautions for laboratory reagents should be exercised while using the reagents. Please refer to Material Safety Data Sheet for detailed information.
Colorimetric Assay Sample treatment: liquid samples such as serum and plasma can be assayed directly. Tissue and cell lysates can be prepared by homogenization in cold 1 x PBS and centrifugation (5 min at 14,000 rpm). Use clear supernatants for assay. Milk samples should be cleared by mixing 600 mL milk with 100 mL 6 N HCl. Centrifuge 5 min at 14,000 rpm. Transfer 300 mL supernatant into a clean tube and neutralize with 50 mL 6 N NaOH. The neutralized supernatant is ready for assay (dilution factor n = 1.36). Note: (1). SH-containing reagents (e.g. b–mercaptoethanol, dithiothreitol, > 5 mM) are known to interfere in this assay and should be avoided in sample preparation. (2). This assay is based on an enzyme-catalyzed kinetic reaction. Addition of Working Reagent should be quick and mixing should be brief but thorough.
1. Equilibrate all components to room temperature. Briefly centrifuge the tubes before opening. Keep thawed tubes on ice during assay. 2. Standards: mix 24 mL 2 mM Standard with 216 mL dH2O (final 200 mM). Dilute standard in dH2O as follows.
Transfer 20 mL diluted standards into separate wells of a clear flat-bottom 96-well plate. Samples: transfer 20 mL of each sample into separate wells of the plate. Note: if a sample is known to contain choline, prepare an extra sample blank well with 20 mL of the sample. 3. Color reaction. Prepare enough Working Reagent by mixing, for each |
|
well, 85 mL Assay Buffer, 1 mL ACHE Enzyme, 1 mL Enzyme Mix and 1 mL Dye Reagent. Add 80 mL Working Reagent to each well. Note: for samples that contain choline, prepare a blank control reagent with no ACHE Enzyme (i.e., 85 mL Assay Buffer, 1 mL Enzyme Mix and 1 mL Dye Reagent). Add 80 mL of the control Reagent to each Sample Blank well.
Immediately tap plate to mix. Incubate 20 min at room temperature.
4. Read optical density at 570nm (550-585nm).
Fluorimetric Assay The fluorimetric assay procedure is similar to the colorimetric procedure except that (1) 0, 3, 6 and 10 mM acetylcholine standards and (2) a black 96-well plate are used. Read fluorescence intensity at lex = 530 nm and l em = 585 nm.
Note: if the calculated acetylcholine concentration of a sample is higher than 200 mM in the Colorimetric Assay or 10 mM in the Fluorimetric Assay, dilute sample in water and repeat the assay. Multiply result by the dilution factor n.
CALCULATION Subtract blank value (#4) from the standard values and plot the DOD or DF against standard concentrations. Determine the slope and calculate the acetylcholine concentration of Sample,
RSAMPLE and RBLANK are optical density or fluorescence intensity readings of the Sample and H2O Blank (or Sample Blank if sample contains choline), respectively. n is the sample dilution factor. Conversions: 1 mM acetylcholine equals 14.6 mg/dL, 0.015% or 146 ppm.
Materials Required, but not provided Pipetting devices, centrifuge tubes, clear flat-bottom uncoated 96-well plates, optical density plate reader; black flat-bottom uncoated 96-well plates, fluorescence plate reader.
Literature 1. Vizi, E.S. et al (1985). A simple and sensitive method of acetylcholine identification and assay. Bioassay combined with minicolumn gel filtration or high-performance liquid chromatography.J Pharmacol Methods. 13:201-211.2. Gilberstadt, M.L. and Russell, J.A. (1984). Determination of picomole quantities of acetylcholine and acetylcholine in physiologic salt solutions. Anal Biochem. 138:78-85.3. | ||||||